Summary
- Karyopharm has a market cap under $1.5 billion, yet peak sales of Xpovio in just its first 3 indications could easily exceed that figure.
- The company has enough cash to easily get the company into 2022 at which time Xpovio sales will likely be well into 9 figures.
- Based on both analyst estimates and my discounted cash flow analysis, Karyopharm looks at least 30% undervalued.
Karyopharm (KPTI) is a commercial-stage pharma company that already markets its leading compound, Xpovio or selinexor, in 2 different cancer indications. Karyopharm fits the model I look for of companies with a novel technology platform that’s already clinically-validated and is trading at a substantial discount to fair value. In this article, I provide an overall view of the company as well as my thoughts on Karyopharm’s valuation.
Novel Pipeline of SINE Compounds with 2 Marketed Indications and Lots of Pipeline Potential
Nearly all of Karyopharm’s approved and pipeline products are considered to be selective inhibitors of nuclear export (SINE) compounds. Cancer cells are masters at manipulating both their internal and external environment to promote their own survival and expansion. One of the ways that they do this is through nuclear export proteins like exportin-1 (XPO1). In ordinary circumstances, XPO1 transports proteins out of the nucleus to prevent levels of them from building too high and having unintended effects. What has been discovered more recently though is that cancer cells can ramp up the activity of nuclear exporters like XPO1 to remove tumor growth suppressors and other beneficial proteins from the nucleus.
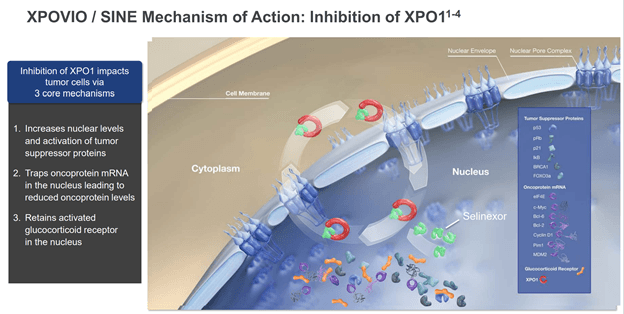
Figure 1: Diagram of how selective inhibition of nuclear export works (source: corporate presentation)
Xpovio and most of Karyopharm’s other assets work by inhibiting the function of these export proteins and allowing levels of important proteins like tumor suppressors to build in the nucleus, which then leads to an overall inhibition of growth. Additionally, the build-up of tumor suppressor proteins in the presence of DNA damage often leads to apoptosis (cell death) of cancer cells but leaving healthy cells that won’t have as high of levels of DNA damage largely unaffected.
Xpovio was approved in July 2019 for penta-refractory multiple myeloma. This is essentially 5th line treatment, after the patient has failed at least 2 proteasome inhibitors, at least 2 immunomodulatory agents, and at least 1 anti-CD38 monoclonal antibody.














